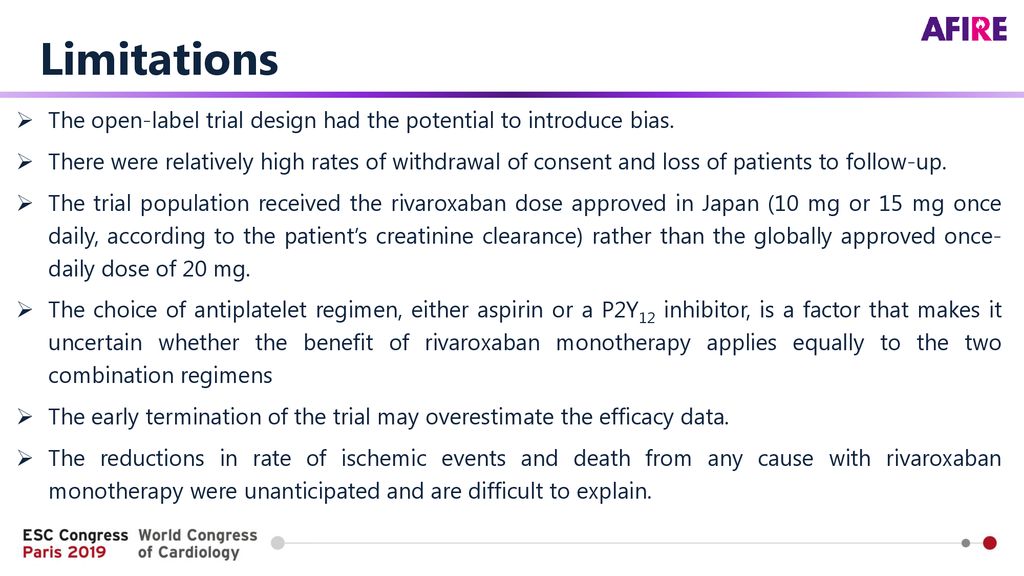
40 open-label study bias
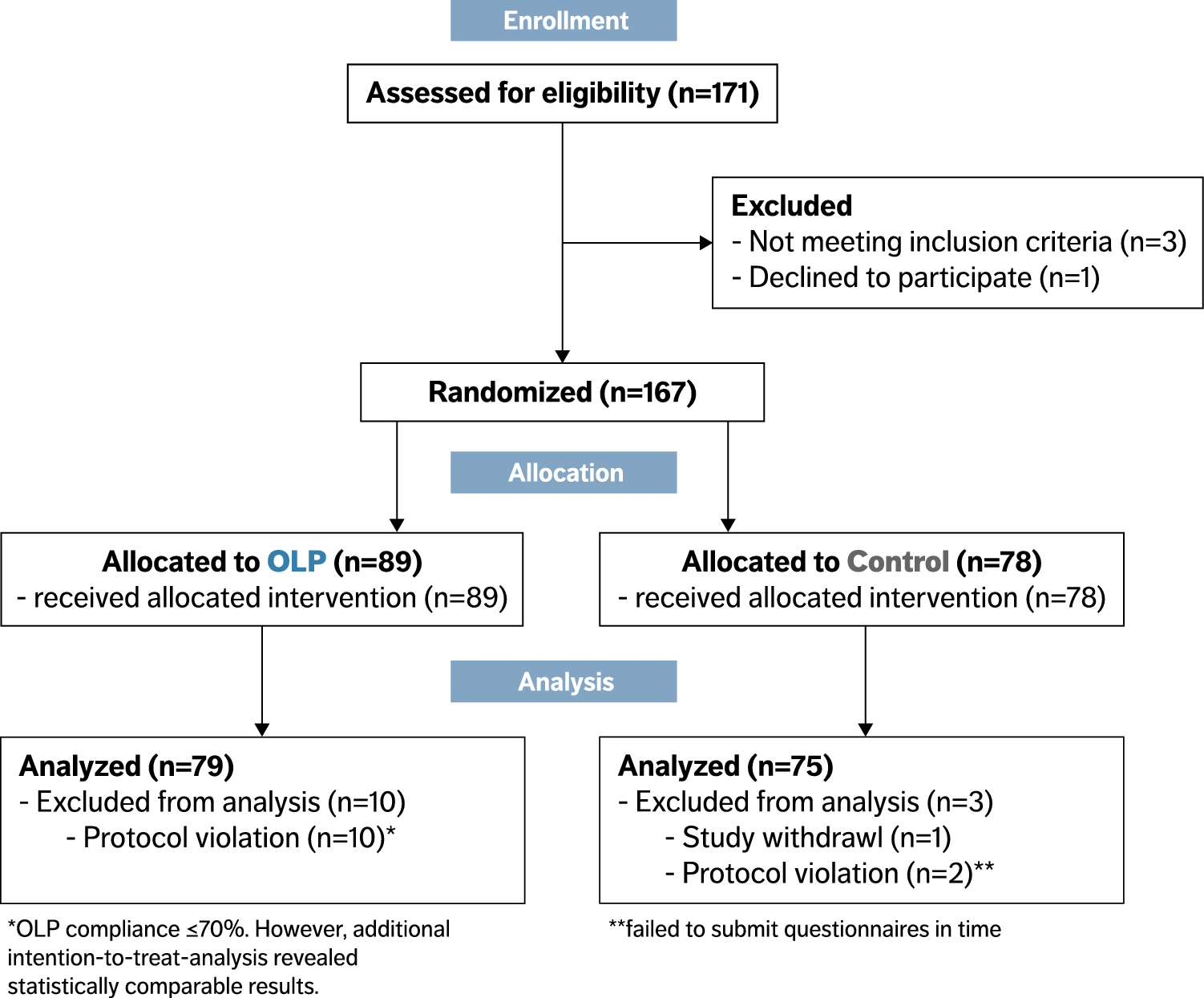
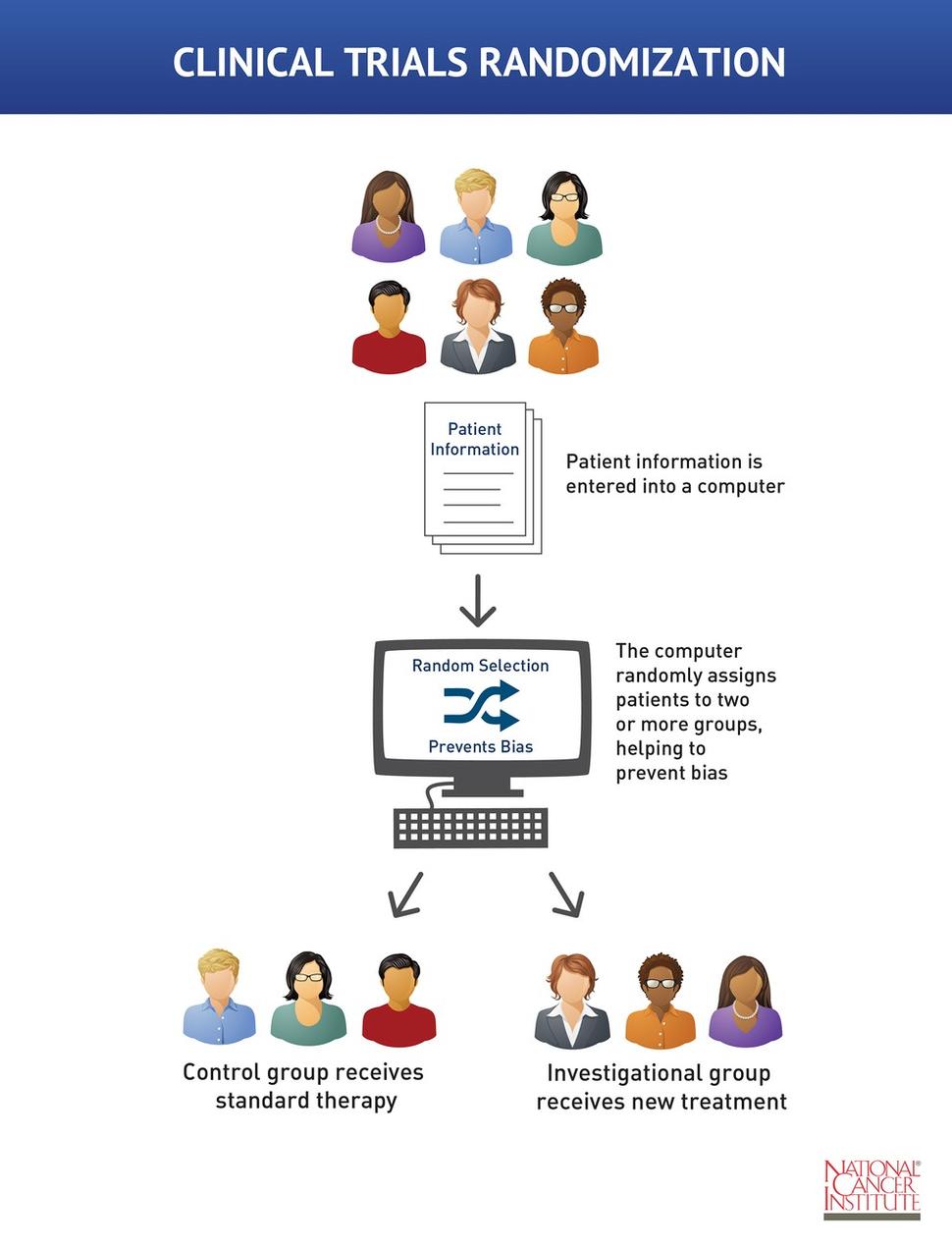
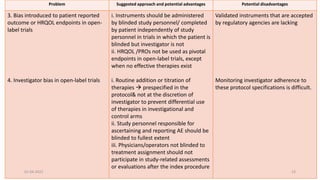
Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [ 6 - 10 ]. Open-Label Extension Studies | SpringerLink Open-label extension studies (OLEs) create a number of challenges for data analysis and interpretation. These challenges include bias in outcomes and adverse clinical events that may exist because of subject selection and participation. OLEs are usually performed following the successful completion of a randomised, controlled clinical trial (RCT) and, thus, the characteristics of the study ...
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG Even for open label studies, it's still desirable blind the study sponsor to reduce potential bias due to the sponsor knowing the treatment level aggregated data while the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in ...
Open-label study bias
Risk of Bias Assessment Forms and Summaries - Psychosocial and ... There are three categories for describing the overall risk of bias for assessed studies: low risk of bias; moderate risk of bias; and high risk of bias. Cochrane Risk of Bias Assessment Tool Use for risk of bias assessments for randomized controlled trials (RCTs). The tool includes seven items in six domains: Selection Bias (2 items) Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology. A Prospective, Randomized, Open Label Trial of Two Doses of Oral ... None (Open Label) Primary Purpose: Treatment: Official Title: A Prospective, Randomized, Open Label Trial of Two Doses of Oral Betaine in Patients With Non-alcoholic Fatty Liver Disease (NAFLD) and Elevated Alanine Aminotransferase (ALT) Actual Study Start Date : November 12, 2013: Estimated Primary Completion Date : December 30, 2021
Open-label study bias. (PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India.... Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. A potential bias in safety evaluation during open-label ... - PubMed A potential bias in safety evaluation during open-label extensions of randomized clinical trials Author Kenneth J Rothman 1 Affiliation 1 Department of Epidemiology, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118, USA. KRothman@bu.edu PMID: 15133780 DOI: 10.1002/pds.943 Abstract National Center for Biotechnology Information National Center for Biotechnology Information
Investigating Potential Bias in Patient-Reported Outcomes in Open-label ... While open-label bias is an important consideration for PRO results, bias related to knowledge of treatment assignment is not unique to PRO and may also affect other common trial outcomes, including progression-free survival 6 and clinician-reported safety data. For instance, the same forces that may lead patients to report fewer or milder ... Survivorship bias - Wikipedia Survivorship bias. This hypothetical pattern of damage of surviving aircraft shows locations where they can sustain damage and still return home. If the aircraft was reinforced in the most commonly hit areas, this would be a result of survivorship bias because crucial data from fatally damaged planes was being ignored; those hit in other places ... Exploring open-label bias in patient-reported outcome (PRO) emotional ... e18702 Background: Concern exists that patients' self-reports may be biased in open-label trial designs. We compared PRO emotional domain results between investigational arms of paired open label and double-blind trials of the same drug and disease population. We hypothesized that greater improvement in emotional domain scores would be found in the investigational arms of open label compared ... National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
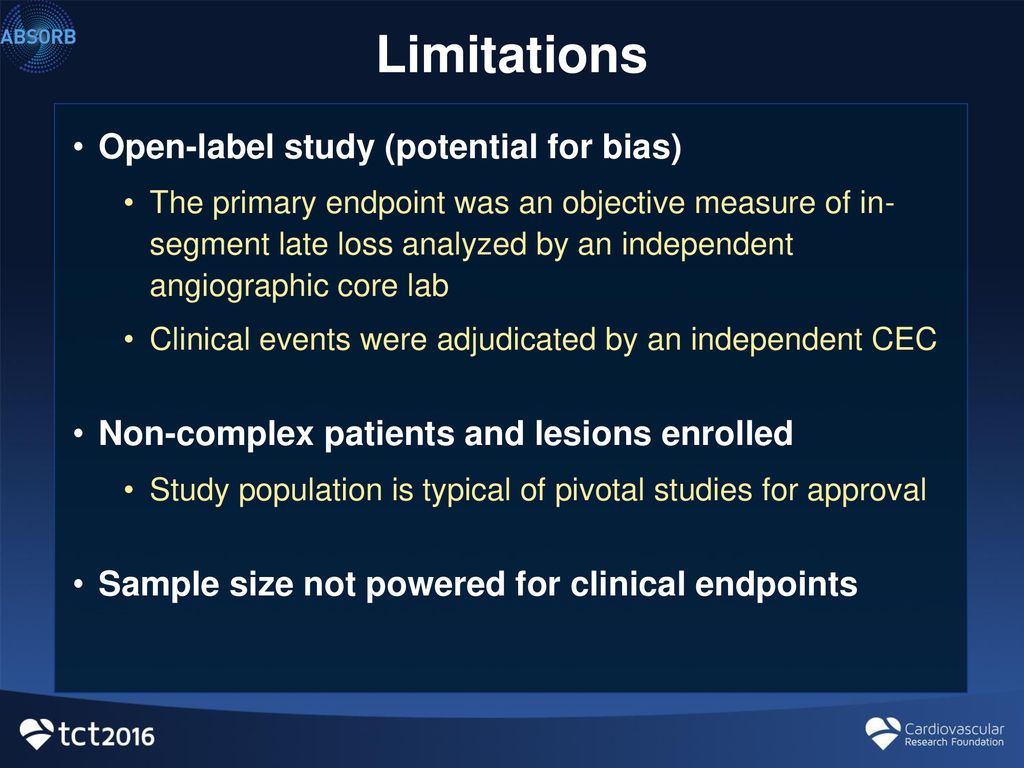
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: • What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,... Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze... PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized
Children and adolescents with ASD treated with CBD-rich cannabis ... First and foremost, this was an open label study, which is likely to create a positive bias in parent and clinician reports given known placebo effects [42, 43].
Open-Label Trial - an overview | ScienceDirect Topics the difference of protective efficacy of ribavirin in humans and in hamsters may have several explanations: (1) the study in humans was not randomized and performed at posteriori, and grouping treated and nontreated patients may have introduced some bias; (2) the dose and mode of infection of niv used for the challenge or the virus replication in …
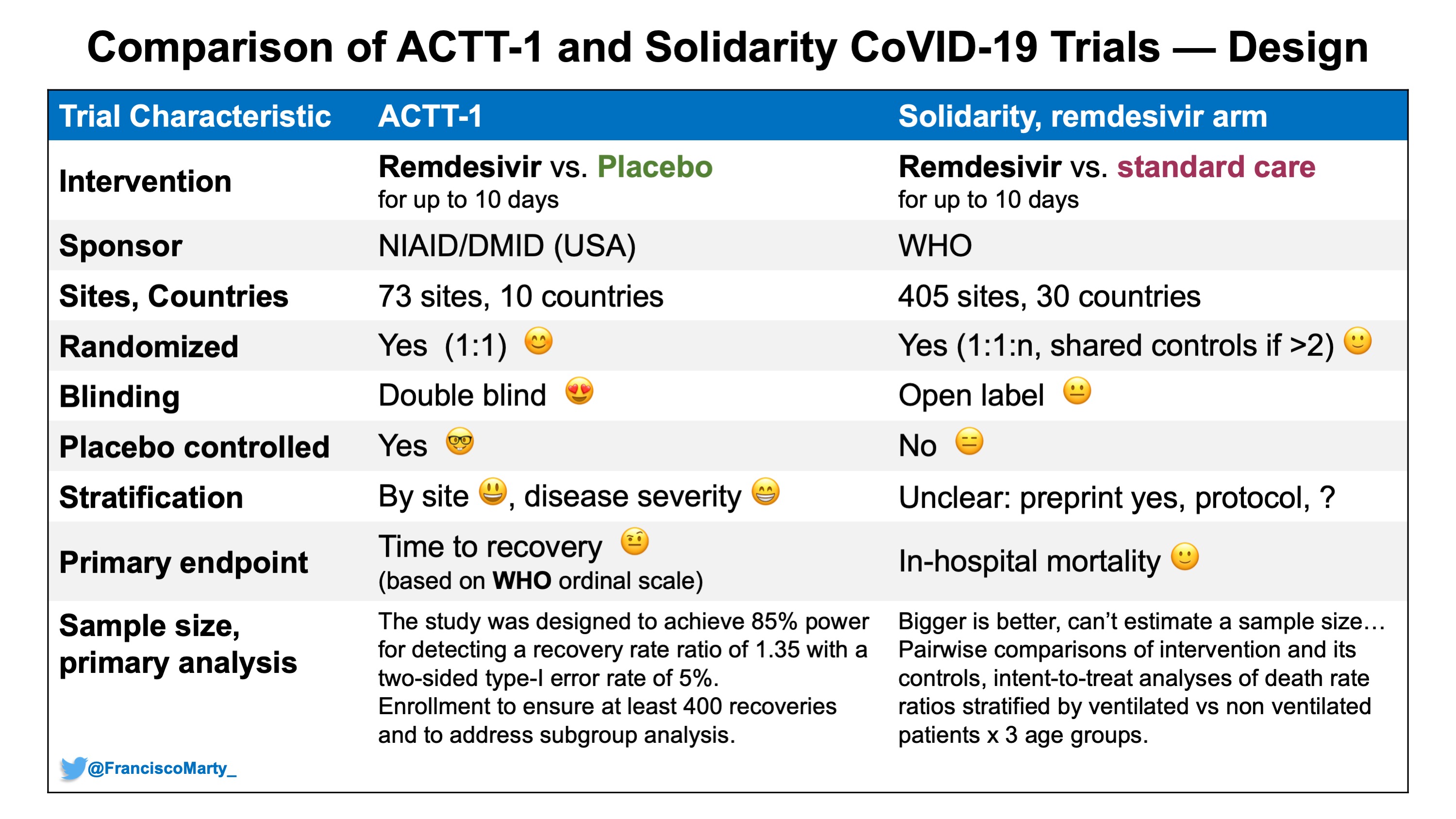
External and internal validity of open label or double-blind trials in ... to the trial design of open-label vs. double-blind double-dummy (Table 1). For instance, while dabigatran was tested in AF using an open-label study [1] and in acute deep vein thrombosis (DVT) and pulmonary embolism (PE) using a double-blind double-dummy trial [2], rivaroxaban was tested inversely with open-label trials in acute DVT and PE [3 ...
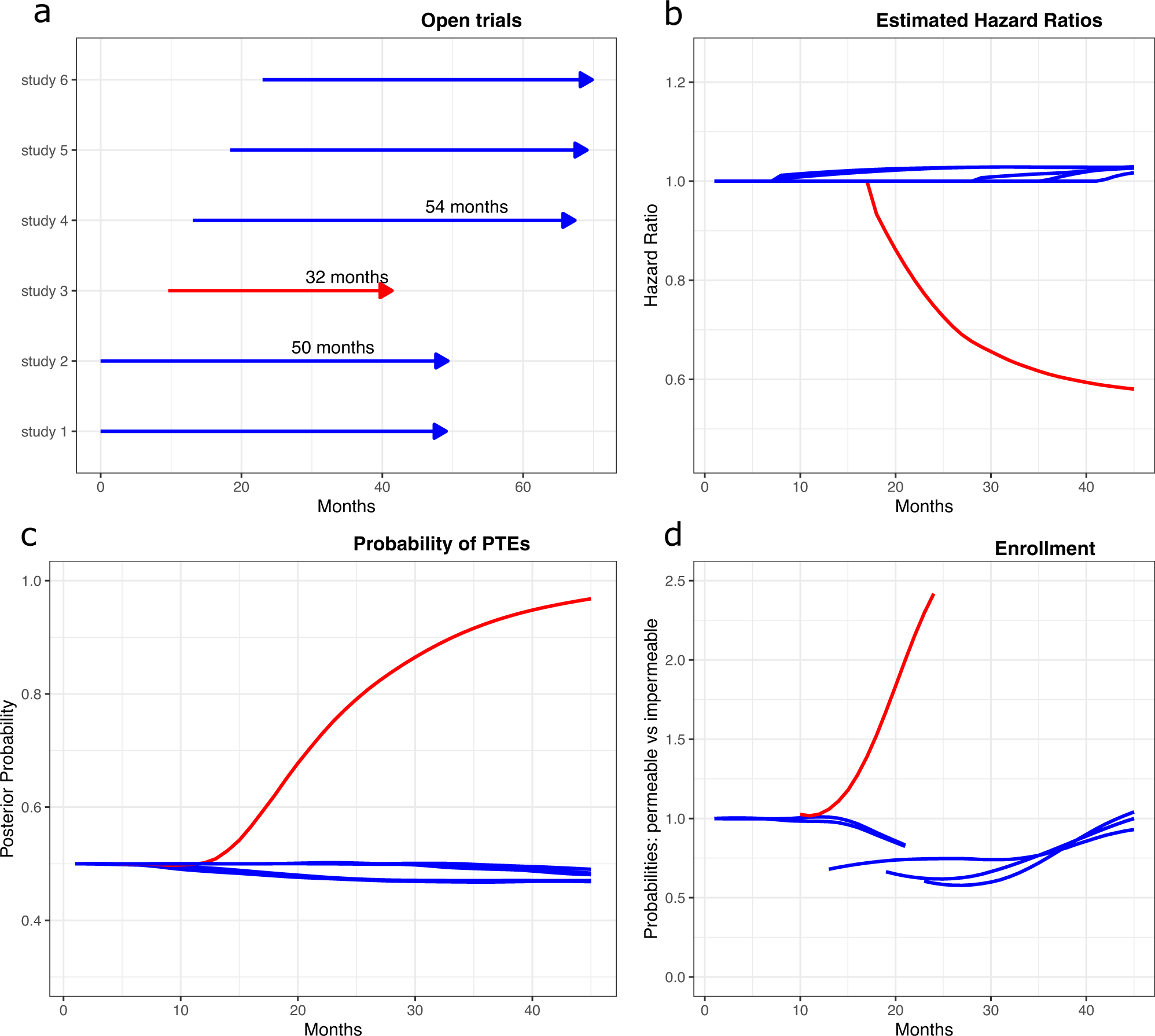
Open label extension studies and patient selection biases Results In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%. Conclusions ...
Consent to open label extension studies: some ethical issues Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. Investigators are typically reluctant to unblind the patients' assignment at the point of entry into the open label phase, on the grounds that this may introduce ascertainment bias in the main study.
Open-Label Extension Studies | SpringerLink The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them. If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label ...
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Open label extension studies and patient selection biases An alternative method of analysis is proposed, which does not rely on the often unjustifiable assumption of outcomes being missing completely at random. Results: In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%.
Open clinical trials | The BMJ Researchers assessed the effectiveness of adenotonsillectomy in children with mild symptoms of throat infections or adenotonsillar hypertrophy. An open randomised controlled trial was performed. Control treatment consisted of watchful waiting. In total, 300 children aged 2-8 years were recruited and randomised to adenotonsillectomy (n=151) or control (n=149).1 The main outcome measures ...
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias.
A Prospective, Randomized, Open Label Trial of Two Doses of Oral ... None (Open Label) Primary Purpose: Treatment: Official Title: A Prospective, Randomized, Open Label Trial of Two Doses of Oral Betaine in Patients With Non-alcoholic Fatty Liver Disease (NAFLD) and Elevated Alanine Aminotransferase (ALT) Actual Study Start Date : November 12, 2013: Estimated Primary Completion Date : December 30, 2021
Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
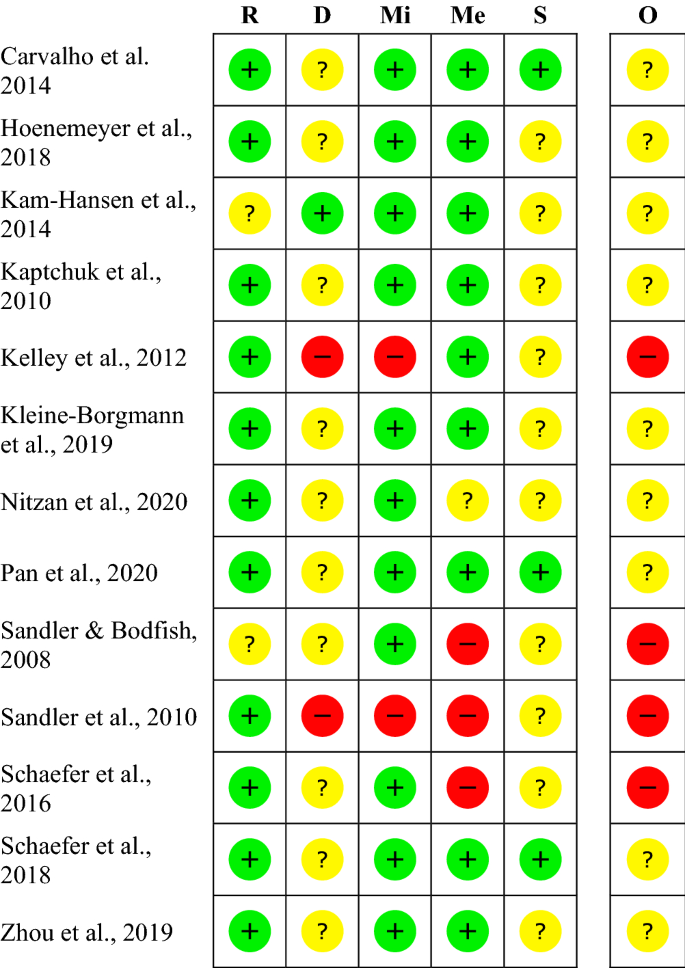
Risk of Bias Assessment Forms and Summaries - Psychosocial and ... There are three categories for describing the overall risk of bias for assessed studies: low risk of bias; moderate risk of bias; and high risk of bias. Cochrane Risk of Bias Assessment Tool Use for risk of bias assessments for randomized controlled trials (RCTs). The tool includes seven items in six domains: Selection Bias (2 items)

























Post a Comment for "40 open-label study bias"