45 what is the term used to label the energy levels of electrons
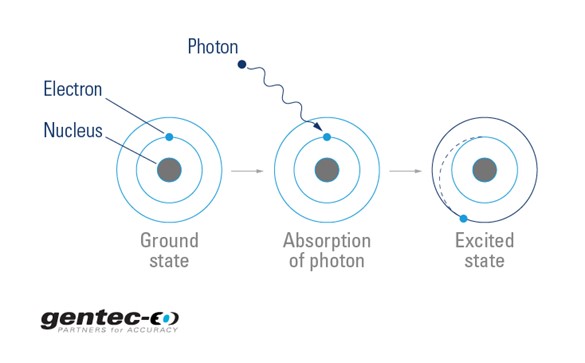
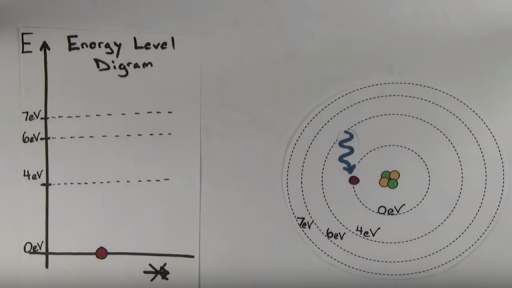
Circle the letter of the term that is used to label the ener - Quizlet Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals b. quantum mechanical numbers c. quantum d. principal ... Atomic Energy Levels (video) | Khan Academy The electron can absorb photons that will make it's charge positive, but it will no longer be bound the the atom, and won't be a part of it. For example at -10ev, it can absorb, 4eV (will move to -6eV), 6eV (will move to -4eV), 7eV (will move to -3eV), and anything above 7eV (will leave the atom) 2 comments ( 12 votes) Upvote Downvote Flag more
The periodic table, electron shells, and orbitals - Khan Academy Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
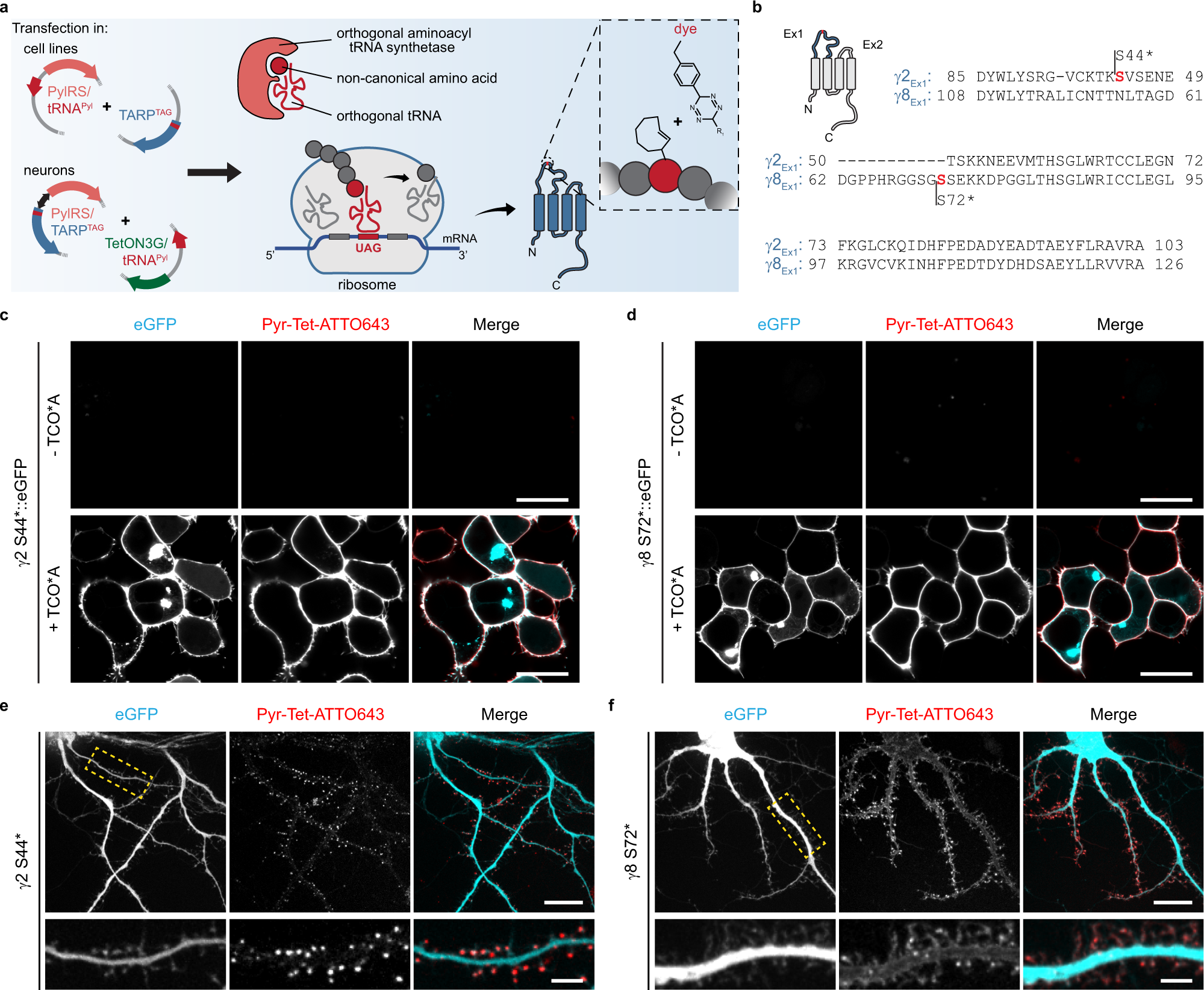
What is the term used to label the energy levels of electrons
Atomic Term Symbols - Chemistry LibreTexts by T Symbols — In electronic spectroscopy, an atomic term symbol specifies a certain electronic state of an atom (usually a multi-electron one), ... What is the term used to label the energy levels of electrons ... It refers to the energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p ... What term is used to label the energy levels of electrons? Nov 13, 2019 · Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital.
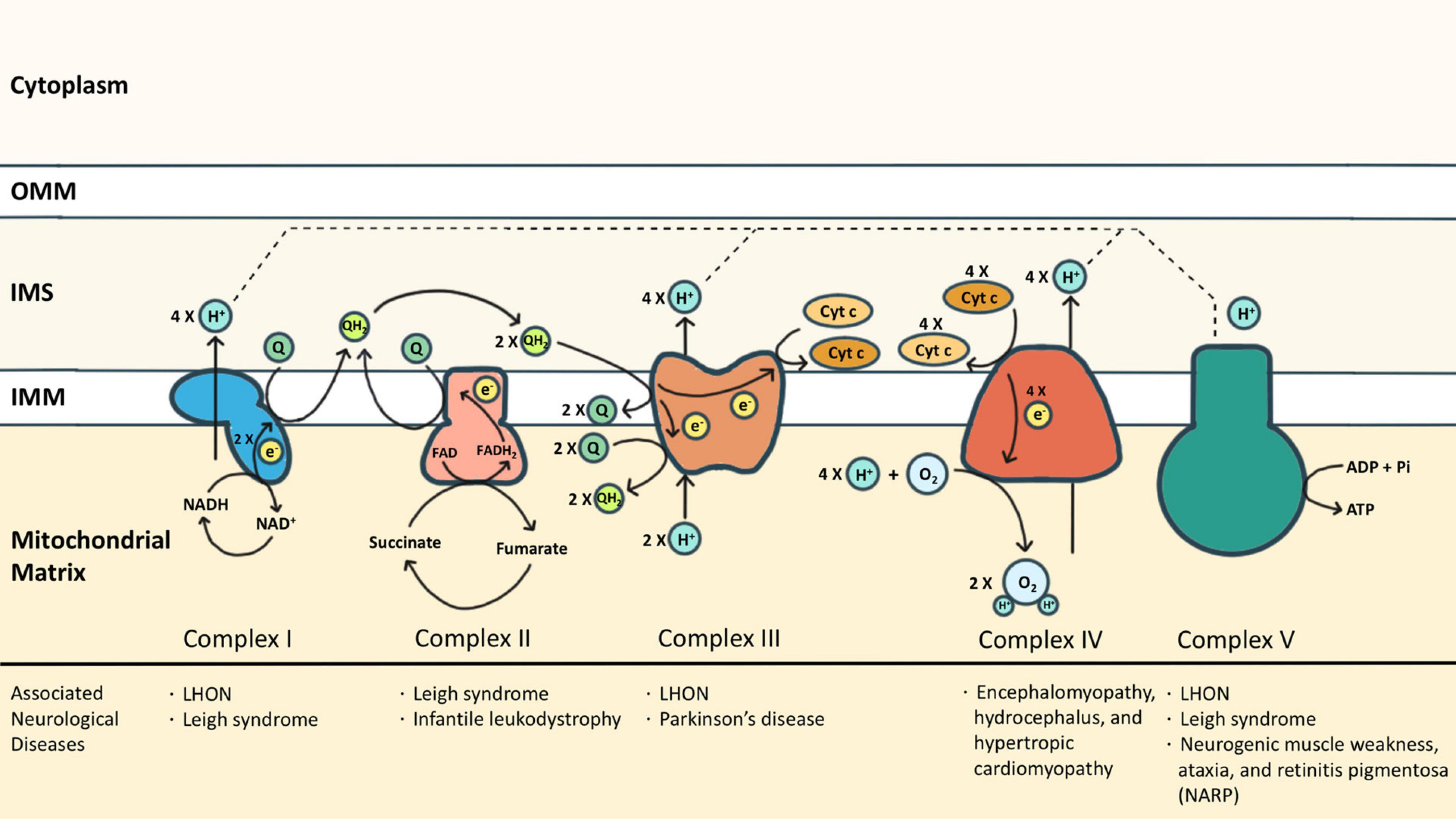
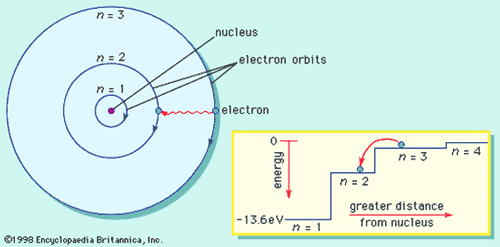
What is the term used to label the energy levels of electrons. Chapter 5 test Section 5.1 Flashcards | Quizlet Nov 4, 2014 · The electrons in an atom can exist between energy levels. Energy levels What are the fixed energies of electrons called? Move an electron from its present energy level to a higher one A quantum of energy is the amount of energy required to? Closer In general, the higher the electron is on the energy ladder, the ______ it is from the nucleus Term symbol - Wikipedia In atomic physics, a term symbol is an abbreviated description of the total spin and orbital angular momentum quantum numbers in a multi-electron atom. Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized . Chapter 5 Electrons in Atoms Flashcards | Quizlet What are the fixed energies of electrons called? energy levels Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. place an electron in an energy level. b. maintain an electron in its present energy level. c. move an electron from its present energy level to a higher one. C
Energy Level | CK-12 Foundation 25 Oct 2021 — Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, ... What term is used to label the energy levels of electrons? Nov 13, 2019 · Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital. What is the term used to label the energy levels of electrons ... It refers to the energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p ... Atomic Term Symbols - Chemistry LibreTexts by T Symbols — In electronic spectroscopy, an atomic term symbol specifies a certain electronic state of an atom (usually a multi-electron one), ...



:max_bytes(150000):strip_icc()/Periodic-Table-Metals-56a12db33df78cf772682c44.png)






















Post a Comment for "45 what is the term used to label the energy levels of electrons"